Category: Medical Devices
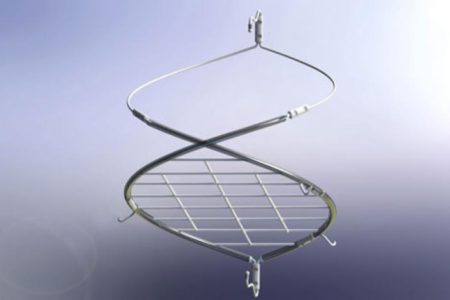
Crux Vena Cava Filter Class Action Lawsuit
Overview The Crux Vena Cava Filter (VCF) is used for the prevention of pulmonary embolism (PE) in patients at risk for blood clots who are unable to take anticoagulant medications. The device consists of a self-expanding Nitinol filter that is...
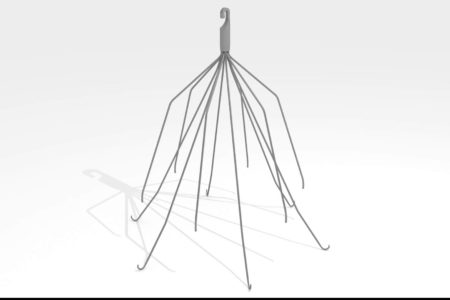
OptEase IVC Filter Class Action Lawsuit
What is IVC Filter Placement? IVC filter placement involves placing a small filter in the patient’s inferior vena cava, a large abdominal vein that returns oxygen-depleted blood to the heart from the lower part of your body. The device is...
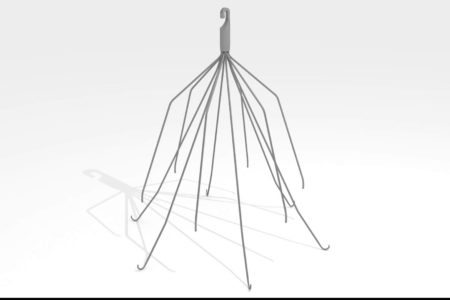
OptionELITE IVC Filter Class Action Lawsuit
Argon IVC Filter Lawsuit Filed in Philadelphia March 3, 2017 – A California woman whose doctor was allegedly unable to retrieve an Option ELITE IVC filter from her body has filed a products liability lawsuit against Argon Medical Devices and...
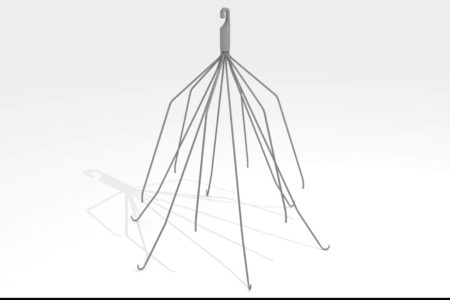
B. Braun IVC Filter Class Action Lawsuit
How Do IVC Filters Work? IVC filters are small, cage-like medical devices that are implanted into the inferior vena cava, a large vein that transports oxygen-depleted (blue) blood from the lower body to the heart. The filters are designed to...
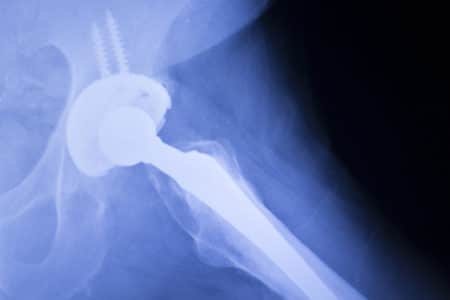
Apex Hip Replacement Class Action Lawsuit
What is the OMNI Apex K2 Hip Implant? The Apex K2 Modular Hip System is made up of a stem, neck and head that can be customized according to each patient’s specific measurements. The implant can provide up to 96...

Congressman Introduces ‘E-Free Act’ to Take Essure Birth Control Off Market
Representative Mike Fitzpatrick (R-PA) has introduced a bill to congress that would force the U.S. Food & Drug Administration (FDA) to take the Essure birth control implant off the market. Free Essure Class Action Lawsuit Evaluation: If you or a...

Essure Class Action Lawsuit
Update: FDA Issues New Guidance For Reporting Essure Problems The FDA has issued new conditions (PDF) to Bayer for submitting post-market information about Essure birth control. Per the updated guidelines, Bayer is required to start providing adverse event reports to...
