Update: Bard Recovery IVC Filters Linked to 27 Deaths, 300 Injuries
September 10, 2015 – A 2-part investigation recently aired on NBC Nightly News questioned why C.R. Bard continued to sell its Recovery IVC filter, even after it was aware that the device was failing at rates significantly higher than competing models. Recovery filters have been linked to at least 27 deaths and 300 non-fatal complications, according to NBC. Click here to learn more.
Overview
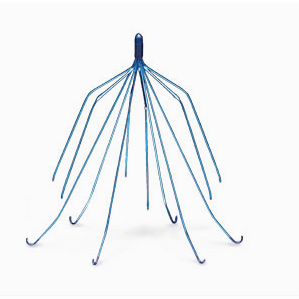
Approved by the U.S. Food & Drug Administration (FDA) in 2002, the Bard Recovery IVC filter is a medical device designed to catch blood clots before they reach the heart and lungs. The filter consists of 12 legs or “struts” (6 short struts positioned over 6 longer struts) which are implanted in patients at risk for pulmonary embolism (sudden blockage of an artery that transports blood to the lungs) and who are unable to take anticoagulant medications. The Bard Recovery is designed so that it can be removed or retrieved once the threat of blood clots has subsided.
What’s the Problem?
Shortly after the Bard Recovery IVC filter hit the U.S. market in 2002, reports of problems with the device began to surface. The filter’s struts can fracture and travel to other parts of the body (particularly to the heart and lungs), where they can cause life-threatening complications.
Signs of an IVC filter fracture may include chest pain and shortness of breath. These symptoms may require emergency medical treatment including cardiac catheterization or CT scan to determine whether the filter has migrated from the implant site.
Bard Recovery IVC Filter Complications
- Fracture of the IVC Filter
- Perforation, Puncture or Serious Damage to the Heart, Lungs or Vena Cava
- Internal Bleeding
- Cardiac or Pericardial Tamponade
- Ventricle Tachycardia
- Lower Limb DVT
- Hematoma or Nerve Injury at the Puncture Site
- Constant and Severe Pain in the Heart, Chest or Elsewhere in the Body
- Pulmonary Embolism
- Infection
- Death
Medical Studies
Medical studies have identified a failure rate of between 21% and 31.7% for the Bard Recovery filter:
- In 2005, a study conducted by the New England Society for Vascular Surgery (NESVS) found a 31.7% fracture rate after examining FDA adverse event data;
- A 2008 study published in the Journal of Vascular and Interventional Radiology (JVIR) indicated a 21% Bard Recovery failure rate, and
- A 2010 study in the Archives of Internal Medicine found that 25% of patients implanted with a Bard Recovery IVC filter had suffered complications, and that 70% of the fractured devices had become lodged in the heart.
Has a Class Action Been Filed?
Bard faces at least 3 class action lawsuits alleging that its IVC filters are prone to fracture and perforate organs. The complaints were filed in California, Florida and Pennsylvania. Allegations raised in the class actions claim that Bard knew for years that their IVC filters were prone to fracture, but that the company failed to adequately warn doctors and patients about this risk.
Do I Have a Bard Recovery IVC Filter Lawsuit?
The Medical Device Litigation Group at our law firm is an experienced team of trial lawyers that focus on the representation of plaintiffs in Bard Recovery IVC Filter Lawsuits. We are handling individual litigation nationwide and currently accepting new injury and death cases in all 50 states.
Free Confidential Lawsuit Evaluation: If you or a loved one has had an IVC filter implanted, you should contact our law firm immediately. Our lawyers are evaluating every individual case regardless of whether you have been injured or not. So, if you have received an IVC filter implant, we would like to speak with you. You may be entitled to compensation by filing a suit against the manufacturer and our lawyers can help.